September 27, 2017
Georgia grows a startup
How Vertera Spine went from university lab to surgical suite
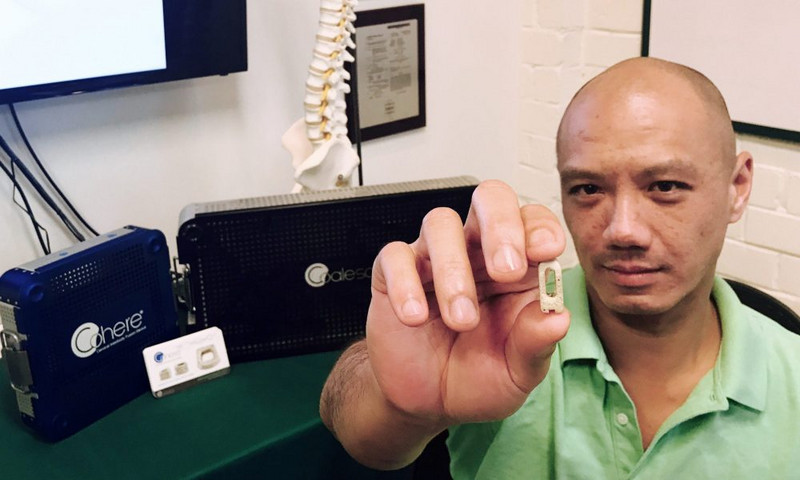
If you want a clearer picture of how GRA’s venture development program works, begin with the happy ending to a good story: the acquisition this month of Georgia Tech startup Vertera Spine.
Vertera Spine, maker of two FDA-approved devices that improve spinal fusion surgery, is now a subsidiary of NuVasive, Inc., a pioneer in minimally invasive spinal technologies. Vertera Spine and its employee base will remain in Georgia for the foreseeable future, according to founder and CEO Chris Lee.
How the deal came to be is somewhat customary. NuVasive had its eye on Vertera Spine for some time, and acquiring the start-up strengthens NuVasive’s position as an innovator in spine surgeries, now estimated to number 400,000 procedures a year.
How Vertera Spine itself came to be is something else — a case study in the workings and impact of GRA Ventures, GRA’s venerable program to drive more discoveries and inventions from the lab to the marketplace.
Rewind to 2012: The Georgia Tech lab of Ken Gall (now at Duke University) invents a way to modify a material called PEEK — commonly used in spinal fusion surgery — so that its surface becomes porous without forfeiting any mechanical strength. To the scientists, the newly invented biomaterial may mean that bones will adhere better to spinal implants after surgery.
Their theory proved to be true following testing on rats in the lab of Bob Guldberg at Georgia Tech. “We’d never seen bone grow into PEEK material like this,” recalls Lee, who collaborated with the Guldberg Lab during his Ph.D. work and had also worked with Ken Gall at another startup, MedShape. “It was a real ‘aha’ moment.”
Enter GRA. An initial grant of $25,000 in 2013 supported the launch of Vertera Spine. The investment allowed Vertera Spine to expand strength testing as well as prepare for U.S. Food and Drug Administration review.
“We used those funds to buy other devices and conduct additional testing at Georgia Tech,” Lee says. “That first grant was essential for getting our first product through FDA approval. If it wasn’t for that, it would’ve taken a lot longer.”
A second GRA Ventures grant helped fund in vivo testing of Vertera Spine’s first implant device made from porous PEEK (which is short for polyetheretherketone, a thermoplastic polymer). Says Lee: “We found that our porous PEEK material performed better than textured titanium in spine surgeries. That’s when we started getting a lot of attention at medical conferences.”
What happened next at Vertera Spine calls to mind pages flying off a calendar:
- November 2016 brought a big award: “Top Ten Best Spine Technologies for 2016” by Orthopedics This Week Magazine.
- A GRA loan in January 2017 propelled Vertera Spine’s product launch of the FDA-approved device Cohere, an implant in cervical spinal fusion operations. Since then, Cohere has been successfully implanted in 2,000 surgeries.
- The next month brought the company’s only equity funding round — $3.1 million invested by 29 individuals (The Company previously raised about $3 million in convertible debt.)
- And summer 2017 brought the green-lighting of an ICD-10 code — which essentially legitimized Vertera Spine’s one-of-a-kind technology for widespread use — as well as FDA approval of Vertera Spine’s second device, Coalesce. Launching this November, Coalesce will be implanted in lumbar fusion surgeries.
Along the way, talks began with NuVasive about a possible acquisition. Startups typically bring in heavyweight bankers to initiate and engage in such negotiations, but Vertera Spine didn’t have to —they had GRA senior advisor Greg Dane to guide them.
“We worked with him throughout the acquisition process,” Lee says of Dane, a Vertera Spine board observer and a seasoned executive who has helped steer dozens of university inventions through the commercialization maze. “Greg was an excellent advisor, so we didn’t have to engage a banker.”
Under NuVasive, Vertera Spine’s porous PEEK technology is poised to transform spinal fusion surgery as a key element of the company’s portfolio of scientifically advanced materials. Lee says the new implants can speed surgical recovery from six to 12 months to six to 12 weeks; that’s because the bone better embraces the implanted material.
And unlike titanium and other metal implants, Vertera Spine’s technology makes it possible to monitor post-surgical progress using more affordable X-rays or CT scans. “So you can assess earlier whether surgery was a success,” Lee says.
According to Ashley Cornelison of GRA, the Vertera Spine story is a textbook example of how GRA Ventures’ investment and guidance plays a key role in helping to grow companies.
“What made Vertera Spine particularly impressive went beyond their compelling technology,” she says. “They have an impressive team of scientists, engineers, industry experts and surgeons who know how to target and reach milestones for their products. And there’s great potential for more products to be developed from their groundbreaking technology.”
