Right Now in Georgia...

Clearside Biomedical pioneers a safer, more effective way to treat retinal disease
Imagine eye disease is robbing you of your sight.
If you leave the condition untreated, you could have permanent vision loss. You might even go blind.
But if you choose one of the most common treatments, you risk developing other eye diseases that could force you to undergo surgery.
It’s a choice no one would want to make. Yet this decision is faced each year by millions of Americans who have diseases of the retina – the area in the back of the eye where images gathered by the eye are sent to the brain.
Clearside Biomedical promises a better choice. Born of research at Emory University and Georgia Tech, the company is developing medical technology that will make the treatment of retinal diseases safer and more effective.
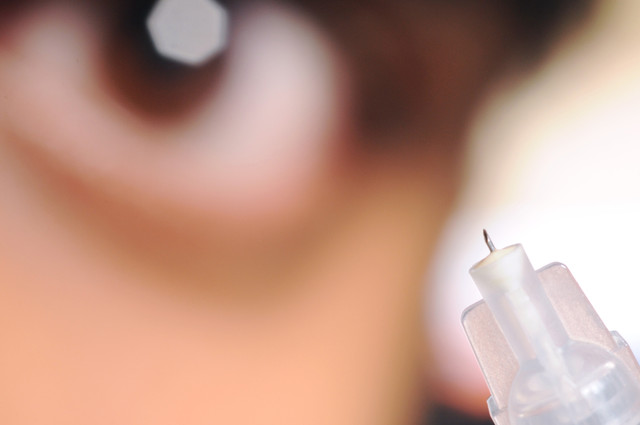
The linchpin in the company’s approach is the use of a microinjection system that uses a tiny needle – smaller in length than the thickness of a coin – to inject medications into a space between the outer eye and the inner eye. Doctors have known for years about this area, called the suprachoroidal space, but until recently, surgery was the only way anyone knew to reach it.
That was until a scientist in Dr. Mark Prausnitz’s lab at Georgia Tech used a microneedle to inject fluid into the space. When he did, the fluid spread throughout the layer, reaching places in the back of the eye that had been nearly impossible to access without surgery.
Yet the injected material didn’t go outside the space into other areas of the eye. It stayed right where it was needed.
Using microneedle injection therapy for retinal diseases would be a huge improvement over current treatment, in which doctors inject steroids into the center of the eye. About 300,000 such injections are given each year in the United States. While steroids help control the swelling, their use is limited because the procedure carries significant risks: More than one-quarter of patients receiving steroid injections develop glaucoma, and nearly three-quarters of patients develop cataracts. Either of these side-effect conditions is likely to require eye surgery.
That’s why ophthalmologists will seize on the new microneedle technology for injections, predicts Clearside Biomedical CEO Daniel White. With the patented microneedle delivery, doctors will be able to perform a simple, non-surgical procedure that delivers medication right to the suprachoroidal space, for safer, more effective treatment.
“The Clearside microinjection platform provides an alternative for retinal specialists to place drug directly on the site of disease and retain drug away from the other compartments of the eye where side effects can occur, and in the case of common steroids, improving the safety and performance to treat sight threatening conditions,” says White, a veteran of biomedical start-ups. “The medicine goes exactly where it’s needed, and stays there for longer periods of time, and since very little reaches other parts of the eye, the risk for cataracts and glaucoma are greatly reduced. This provides another tool for retinal specialists to treat conditions like macular edema and adult macular degeneration, which lead to blindness.”
Better treatment is essential, as health experts predict incidences of retinal disease will climb substantially in the next 40 years. In particular, experts are concerned about eye disease caused by diabetes. About one-fourth of people who have diabetes for 20 years or more will develop macular edema, a swelling of the retina that causes the very center of the visual field to become blurry or filled with blind spots. Where a person’s vision should be sharpest, it is instead useless.
Today, diabetes-related eye disease is the leading cause of blindness among working-age adults, with more than 10,000 people losing their sight in the United States each year. As diabetes explodes among the U.S. population, the incidence of eye disease will also increase. Today, more than one million Americans have diabetes-related eye disease severe enough to threaten their vision; health experts predict that number will grow to 3 million by 2050.
That means the need for Clearside Biomedical’s product also will grow. The company’s business plan impressed the investors at Hatteras Venture Partners, who called the technology “highly innovative, based on elegant science,” and backed up that praise with a $4 million investment in January 2012.
The following month, GRA Venture Fund and the University of North Carolina’s Kenan-Flagler Private Equity Fund added supporting capital, which White says was important to the company’s early development.
"Any start-up company is a combination of founders, technology and capital,” he explains. “The capital is important, but the people behind the capital are also important. Being plugged into the GRA network is of great benefit. They connect us with talent and experienced service providers, and strengthen our relationships with Georgia’s top universities, which may lead to potential future opportunities and other technology to help us grow."
Early in 2013, Clearside received a $7.9 million investment in a second round of funding. By the end of the year, the company began closing in on completion of its safety trials. And the company’s initial product – a microinjection-based steroid – is expected to hit the market within five years.
As the company grows, its biomedical and commercial operations will be headquartered in Atlanta.